BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://nmj.umsha.ac.ir/article-1-2038-en.html
2- Associate Professor, Ph.D. in reproductive health, School of Nursing and Midwifery, Hamadan University of Medical Sciences, Hamadan, Iran ,
3- Associate Professor, Faculty of Health, Hamadan University of Medical Sciences, Hamadan, Iran
✅ The Bristol tool is a good tool for evaluating mothers breastfeeding.
Today, the importance of exclusive breastfeeding in reducing infant mortality has been acknowledged [1]. The World Health Organization recommends exclusive breastfeeding for the first six months of the baby's life and continued breastfeeding with solid food for up to 2 years [2]. Breast milk not only reduces neonatal disease (sudden death syndrome, respiratory, gastrointestinal and ear infections) and childhood (allergy, asthma, obesity), but also reduces ovarian and breast cancer rates in the mother [3].
Assessment of breastfeeding requires tools to assess the way of breastfeeding and provide more effective counseling to the mother. One of these is the Bristol Breastfeeding Assessment Tool (BBAT), which contains 4 items (breastfeeding status, sticking of the neonate to the mother, Swallowing and suckling). This tool can easily assess the level of maternal need for help and counseling in breastfeeding [7]. There are several factors that have a negative effect on breastfeeding continuity, one of which is cesarean delivery. Cesarean mothers need more support comparing to mothers who have had a normal childbirth, especially in terms of proper breastfeeding [8]. Breastfeeding support programs are offered in a variety of ways. One of the most effective methods used by health care providers to solve clients’ problems is counseling [9].
Since no Bristol tool has been used in Iran so far and considering the importance of identifying breastfeeding problems, the aim of this study was to use Bristol Tool (BBAT) in breastfeeding counseling and its effect on exclusive breastfeeding status and its frequency in primiparous cesarean mothers.
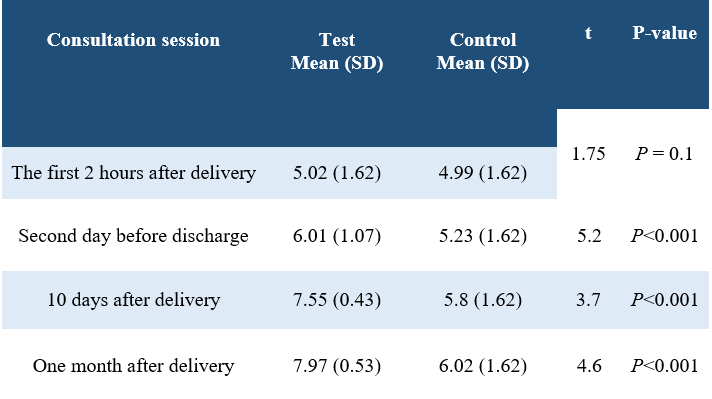
This study is quasi-experimental with pre-test and post-test design. A total of 80 primiparous cesarean women in Fatemieh Hospital, Hamadan, Iran in 2018 (40 patients in each of the control and test groups) were recruited by convenience sampling method (couple days in the test group and individual days in the control group). Inclusion criteria included cesarean delivery, preterm delivery, term and singleton infants, and no banning for neonate for breastfeeding. Exclusion criteria were the mother's unwillingness to continue working, the woman's continued absence from attending at least one breastfeeding consultation, and her ability to not breastfeed. Sample size, which was 1% with type 1 error and 90% study power, was calculated for 40 subjects in each control and test group. Sampling was done from April to September 2018. At the first visit with the mother during the first two hours after delivery, the mothers were given explanations on the subject and the manner of doing the research and informed consent was obtained from them to enter the research and fill out the questionnaire form. Demographic information questionnaire was completed in two groups of control and test. In both groups, breastfeeding was assessed by the researcher using the Bristol tool. Before the study, both control and test groups had no significant difference in the scores of the Bristol assessment (P=0.1).
In two groups, the researcher reviewed the Bristol Breastfeeding checklist, assessed the breastfeeding status, nuchal infant, milk swallowing and infant suction and, based on the need, a breastfeeding counselor was provided in the test group. The counseling was given to the mother during the first 2 hours, 2 days, 10 days and one month after delivery. During this time, the control group received routine care only. The lactation continuity questionnaire was completed for both groups during the four months after delivery.
After data collection, descriptive statistics were used to summarize the data. The central and dispersion indices were used to describe the quantitative variables. Independent t-test, paired t-test, and Chi-square test were used to compare the characteristics of the two groups. Normality was assessed by Kolmogorov-Smirnov test, which confirmed the normality of the data. SPSS 23 (SPSS Inc., Chicago, IL., USA) was used for data analysis and the significance level of tests was considered to be less than 5%.
Two groups had no significant differences in demographic variables (P>0.05). Four months postpartum exclusive breastfeeding showed a significant difference between the two groups (P=0.04) (Table 1). There was a significant difference between the mean duration of lactation during the four months postpartum (P<0.001) (Table 2). The mean of Bristol instrument scores in the first session did not have a significant difference between the two groups, but in the second, third and fourth sessions after delivery, there was a significant difference between the two groups (P <0.001) (Table 3).
Table 1. Comparison of breastfeeding status of mothers during the four months after delivery in the control and experimental groups

Table 2. Comparison of the mean score of breastfeeding continuity during the four months after delivery in the control and experimental groups.
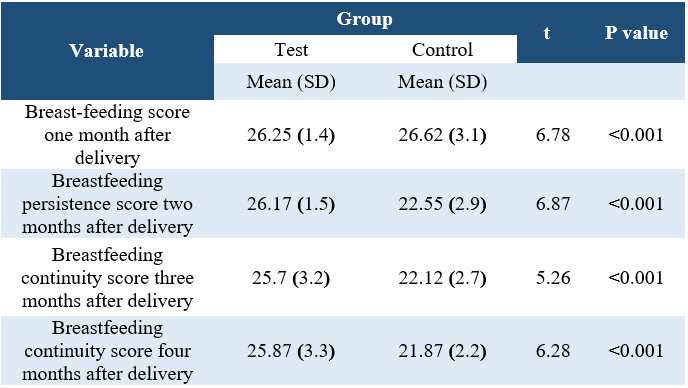
Table 3. Comparison of the mean score of the total Bristol tool in the four counseling sessions in the two groups
The aim of this study was to use the Bristol Tool (BBAT) in breastfeeding counseling and its effect on the status of exclusive breastfeeding and its frequency in primiparous cesarean mothers. The results showed that after counseling, there is a significant difference in the exclusive breastfeeding of mothers. Saba and colleagues found face-to-face education is effective in increasing mothers' awareness and performance in breastfeeding [10].
This study was carried out only in one health center due to the limitation of public delivery centers in Hamadan. On the other hand, the limited number of samples can reduce the generality of the results of this study. It should be noted that, long-term follow-up of more than 4 months postpartum was not possible due to limited funding in this study. It is recommended that further research be conducted with a larger sample size, more birth centers and longer follow-up.
The Bristol tool is a good tool for evaluating mothers’ breastfeeding.
The clinical trial code of this study is IRCT2017041810426N16 and the code of ethics is IR.UMSHA.REC.1396.66. The researchers express their gratitude for the sincere cooperation of the mothers and staff of Fatemieh Hospital in Hamadan and Hamadan University of Medical Sciences for their financial support.
The authors declared no conflict of interest regarding the publication of this article.
Received: 2019/05/18 | Accepted: 2019/06/6 | Published: 2019/08/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







 gmail.com
gmail.com